The search for lower energy desalination technology continues. Do biomimetic membranes - mimicking the chemistry found in nature — hold the answer? Dr Graeme K Pearce explains how the technology works and looks at two efforts underway in Denmark and New Mexico.
Membranes provide a selective barrier in separation processes. For water treatment, the goal is usually to allow water to pass, while retaining contaminants. There are two classes of membrane technology used in water treatment, namely membrane filtration and reverse osmosis (RO). Membrane filtration uses a fine porous membrane barrier, whereas reverse osmosis uses a dense non-porous active layer film on a porous support.
In membrane filtration applications, the membrane acts as a fine sieve and the contaminants are micro-organisms or fine suspended silt particles causing turbidity. In desalination applications, RO membranes are used to reject salt but allow water to pass through. RO membranes are dense or non-porous, so the water has to dissolve in the film layer and is transported across the film due to the applied driving force.
Membrane filtration has been enthusiastically adopted by the water industry during the last 15 years. The membrane barrier essentially provides complete removal of any particles larger than the pore size of the membranes, so it achieves a predictable and reliable output.
Energy requirements are similar to conventional treatment technology, and since this is low in comparison to the energy required for distribution and transport (1), the water industry has been keen to upgrade its treatment capability.
Unfortunately, the energy required for recovering water from saline sources is significantly higher. Membrane desalination technology has been widely implemented out of necessity where alternative resources are not available and the only other option is long distance transfer. However, the water industry recognises that seawater desalination could not be a general answer to the world's impending water crisis, since the energy cost would be unacceptable.
Wastewater reuse offers a compromise by providing a resource at a much lower energy cost than seawater (2) and large scale schemes have already been implemented in Singapore, California and elsewhere. However, these schemes are not always possible and the search for lower energy desalination technology has continued.
Membrane modification
Ever since commercial industrial membranes were first manufactured a century ago, scientists have sought ways of modifying them. A membrane based on a single polymer normally has low permeability and it is difficult to control the selectivity. Adding co-polymers during the manufacturing process allows the properties of the membrane to be adjusted and post treatment can allow further fine tuning. Today's commercial ultrafiltration (UF) and microfiltration (MF) membranes combine high permeability with an impressive selectivity by the use of additives.
In water treatment, it is common to form UF membranes with a pore size of between 10 and 20 nm (nanometers), while MF has pores of between 40 and 100 nm. These membranes are effective in different feed types. Below 10 nm, it is found that membrane permeability drops rapidly. By the time a membrane reaches a rating suitable for RO, effectively < 1 nm, it no longer acts as a fine sieve at all, and the active layer of the membrane is described as dense. Water is not readily transported though the 'pores' of an RO membrane and high driving pressure is used to force the water through.
The idea behind biomimetic membranes is to use or mimic the chemistry found in nature to facilitate water transport though the membrane so that it effectively acts as a fine sieve. In this case, the contaminants rejected would be much smaller than fine particle size and would be dissolved in water. However, the pores would be small enough and have the appropriate charge properties to reject these species, but still facilitate the transport of water across the membrane barrier without the resistance intrinsic to the RO process. The molecules used in nature to facilitate water transport though cell walls are proteins known as aquaporins.
Biomimetic membranes based on aquaporins
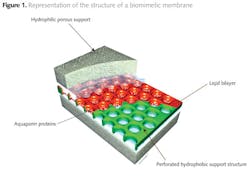
A Danish company called Aquaporin, together with the Danish Technical University nanotech Department, has pioneered the development of biomimetic membranes utilizing aquaporins (3,4). Figure 1 shows the development of supported biomimetic membranes by inserting aquaporin protein molecules to act as water channels between hydrophilic porous supports. The membrane itself imitates the structure of a cell wall by forming a lipid bi-layer, with the aquaporins used to form the bridge through which water transport can occur.
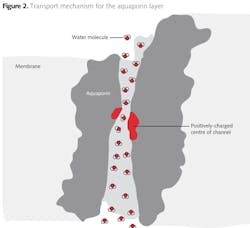
The aquaporins form fine 'channels' across the separating layer of the membrane, but the mechanism by which ionic species are rejected and retained on the feed side are electrostatic repulsion rather than size exclusion. The highly permeable channels present low resistance to the passage of water molecules, while the ionic species are selectively rejected by charge rejection, as illustrated in figure 2 (5).
The aquaporin layer forms a bridge across the lipid bi-layer. This allows a claimed permeability improvement, relative to an RO membrane of one to two orders of magnitude. Under these conditions the energy required for purifying water will drop significantly, potentially making desalination a much more widely applicable technology. Although it is likely that fluxes will still be modest, low operating pressures could make the technology commercially attractive due to low energy cost.
The challenges for aquaporin membranes will be robustness and longevity. The support needs to be kept clean and free from particle fouling. Cleaning procedures are well established in membrane filtration, but they might be too aggressive for the aquaporin layer. Furthermore, the layer may exacerbate fouling effects from concentration polarisation.
Synthetic biomimetic membranes
A second approach to biomimetic membranes is to imitate nature by constructing an inorganic water channel. Rather than use aquaporin proteins, Sandia Laboratories in New Mexico has synthesised water channels from inorganic materials which perform the same selective functions (6). Using nanotechnology methods, Sandia has constructed an inorganic membrane from silica which can achieve the same charge repulsion mechanism as aquaporins, while facilitating the transport of the water molecules themselves.
Using Atomic Layer Deposition (ALD), the membrane is built up one molecular layer at a time to provide a structure which is precisely tuned in terms of both pore size and surface chemistry. Transmission electron microscopy is used to check the structure and ensure than the required pore size is achieved of around 1 nm. Furthermore, Sandia claims to modify selectivity so that rather than just rejecting all ionic species, the rejection can be tailored so that some ions, eg divalent ions, can pass. This has the potential for making the product suitable for drinking water purposes without post-treatment, as sometimes required by RO.
Membranes produced so far show promise, but the Sandia project is still at the research stage and further work is planned to optimize pore channel dimensions and characteristics and demonstrate commercial potential.
Conclusions
• Membranes have been widely used in the water industry, but desalination remains too energy intensive for general application, apart from in arid areas where the only alternative in long distance transfer
• Biomimetic membranes show promise to reduce the energy requirements of desalination significantly by using charge repulsion to reject ionic species while facilitating the transport of water molecules
• Work in Denmark has shown that aquaporin proteins, which are used in nature to achieve transport of water through cell walls, can be incorporated into membrane structures to achieve promising separation with very high permeability
• Commercialization of this technology is in its infancy, and although robustness and longevity still need to be established, the concept shows potential for addressing the energy problem of desalination
• Synthetic biomimetic membranes have been made from silica at Sandia Laboratories in New Mexico and may provide an alternative approach to the same concept, but this work is still in the research phase.
References
(1) Nanofiltration: New Developments Show Promise, G K Pearce, WaterWorld, Vol 26, issue 3, June/July 2011
(2) Water-Energy Interactions of Water Reuse, Edited by K-H Choo, V Lazarova, P Cornel, IWA Publishing, ISBN: 97818433954 16, 2012
(3) http://www.nanotech.dtu.dk/Research/Researchgroups//Bioanalytics/Projects/Biomimetic membranes
(4) Borgnia M; Nielsen S; Engel A; Agre P. 1999. Cellular and molecular biology of the aquaporin water channels. Annual Review of Biochemistry. 68(1): 425-458
(5) Global Water Intelligence Water Market Report, 2010
Author's note: Dr Graeme K Pearce is a membrane technologist with 25 years experience in the membrane industry. He is principal at Membrane Consultancy Associates — a consultancy for providing advice and guidance in membrane technology for liquid separators. For more information please email: [email protected].
More Water & WasteWater International Current Issue Articles
More Water & WasteWater International Archives Issue Articles