Chromium is a metal found in nature within certain geological formations, often in natural deposits of ores containing other elements. In addition, it is generated from human manufacturing activities, such as in steel making production and in metal plating processes. The trivalent form is a required nutrient, while hexavalent chromium (also identified as Cr(VI) or Chromium-6) is a known carcinogen and an emerging health concern in potable water sources.
Hexavalent chromium is currently regulated throughout the United States under a federal drinking water standard for Total Chromium set at 0.1 mg/L (100 ppb). More stringently, the State of California has established its Maximum Contaminant Level (MCL) for Total Chromium at 0.05 mg/L (50 ppb). However, in early 2024, it is anticipated that California, through the State Water Resources Control Board Division of Drinking Water, will promulgate a new MCL specifically targeting hexavalent chromium at 0.01 mg/L (10 ppb). Once enacted, the larger water utilities in the state will be required to be in compliance with the new standard by 2026.
Hexavalent Chromium Occurrence in California
In California, approximately 40% of the total water supply originates from groundwater (SWRCB, 2023). California’s reliance on groundwater continues to increase as a result of drought conditions, changes in surface water availability, and increases in demand by municipal, agricultural, and industrial users.
As of recent 2022 data, 501 sources (of 233 public water systems) in California have potable water sources with hexavalent chromium concentrations above the proposed MCL. These sources serve nearly six million residents, and the new standard has been correlated to the prevention of approximately 900 cancer cases over 70 years statewide (SWRCB, 2022).
Hence, for such community water systems that are heavily reliant on groundwater sources for their potable water supply, the need for treatment of hexavalent chromium is of paramount importance.
Treatment of Hexavalent Chromium
To meet the pending upcoming regulation and provide the health benefits to the affected residents, a number of potable water treatment technologies for hexavalent chromium are available, including physical/chemical, chemical, and biological processes.
However, albeit on a limited basis to date, traditional resin based treatment technologies have already been implemented and permitted in California, including both strong base anion (SBA) exchange and weak base anion (WBA) exchange systems.
The ultimate choice of any treatment technology remains a function of the feedwater quality and chemistry. Critical water chemistry factors affecting treatment include the concentration levels of Cr(VI), alkalinity, nitrate, sulfate, total organic carbon and other metals. In addition, treatment selection is a function of the operational usage of the system, associated capital equipment costs and operational expenses.
The operational expenses are treatment approach specific, and include chemical usage, brine waste generation, waste disposal and operator attention required. All of these factors result in a total expenditure cost that a water utility will expect to pay for such treatment.
Strong Base Anionic Resin Treatment Systems
The use of strong based anionic resin has advantages for challenging feedwater streams. The affinity for SBA resins for metals and oxyanions is:
CrO42– --> SO42– --> NO3– --> Cl– --> HCO3–
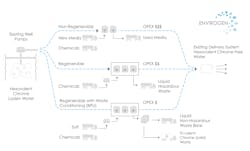
Typically, where nitrate and sulfate concentrations are low to moderate, such feed water chemistry often affords this technology approach with defined benefits. The use of such resin can occur in non-regenerable single-pass ion exchange (IX) systems, regenerable IX systems without brine processing, and regenerable IX systems with brine processing (Figure 1).
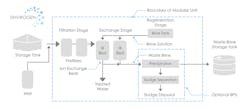
A typical schematic of these SBA IX configurations is provided (Figure 2). Such systems have prefiltration to remove solids, multiple IX vessels (either in series or parallel) to treat the chrome, on-line instrumentation (i.e., flowmeters, analytical meters, etc.) to provide feedback data to control the regeneration sequence if required, and, in some cases, disinfection as post treatment.
Non-Regenerable Single-Pass IX SBA Systems
With amenable water chemistry (e.g., low sulfate concentrations), several thousand bed volumes (BVs) of resin capacity can be achieved using single pass SBA systems. Ultimately, this resin will become exhausted, requiring removal and replacement.
This approach is often beneficial for utilities that intend to treat a water source on an intermittent or “as needed” basis, leading to lower media change-out frequencies. However, the resin will also treat other metals like arsenic and uranium. Hence, when replacement is required, consideration must be given regarding the characterization of the resin as a solid waste in terms of ultimate disposal.
Regenerable IX SBA Systems without Brine Processing
IX systems using SBA can be regenerated on site using a concentrated salt solution (i.e. sodium or potassium chloride), typically in a counter-current flow process. Such an approach is beneficial when competing oxyanions of sulfate or nitrate are in larger concentrations which compete for available bed volume capacity of the resin.
Such on-site regeneration allows for continuous operation but, with the generation of a waste brine, offers an operating cost of salt usage and brine disposal. The waste brine can also contain levels of metals that have been removed in the regeneration process, potentially classifying the brine as a hazardous waste in California. Such costs for disposal need to be accounted for when evaluating this process versus providing a brine processing unit (BPU).
Regenerable IX SBA Systems with Brine Processing
For waste brine that could be classified as hazardous, a BPU can be used to process the brine through chemical reduction and precipitation processes, reducing Cr(VI) ions to Cr(III) which then precipitates out as chromium hydroxide solid.
The solid material is then sent to a dewatering tank where it further solidifies, producing 2 to 3% solids waste. Overall, such a process removes the metals that contribute to the hazardous classification, resulting in the waste brine being nonhazardous for off-site disposal. This approach requires another unit operation as capital equipment, chemicals to reduce and precipitate out the metals, and an additional level of operation attention.
Weak Base Anion Exchange Systems
Similar to non-regenerable single-pass IX SBA systems, WBA resin systems typically use a single use approach of loading and disposal. WBA resin systems are often used where sulfate levels are too high for SBA use, with a lead-lag or lead-lead-lag vessel approach.
In order to achieve large bed volume capacity of the resin (100,000+ BVs), a reduction in the influent water pH to a range of 5.0 to 6.0 is required. This pH reduction facilitates the hexavalent chrome treatment and can allow for long periods between resin changeout. One concern for this approach is that, after treatment, additional pH adjustment chemicals are required to raise the pH again. Like all single pass resins, other metals will be removed, so the WBA resin will need to be properly characterized before disposal.
Additional Regulatory Oversight Concerns
All municipalities and utilities implementing hexavalent chromium treatment in California will be required to interact with a number of state regulatory agencies. The Division of Drinking Water will oversee and permit the use of the system for potable water treatment.
However, due to the hexavalent chromium concentration on the resin, or eventually in a waste brine solution, a number of other agencies will oversee aspects of permitting the plant for operation. The Department of Toxic Substances Control, the local California Unified Program Agency (CUPA), and possibly even the local fire department will potentially be involved as the system is permitted. The BPU process generally will be defined as a Conditionally Exempt-Small Quantity Treatment per CUPA.
With a number of agencies involved in overseeing this treatment, utilities should plan accordingly as they develop project implementation schedules for these systems.
Conclusion
Regulation of hexavalent chromium in California should become a reality in 2024, and utilities need to begin to assess their water source treatment needs to meet this pending mandate. A number of technologies exist for the treatment of Chromium-6, but a thorough understanding of the water chemistry and operation are required to refine the optimal system selection. Both SBA and WBA ion exchange systems can be effective for hexavalent chromium treatment. Still, variations of this approach exist, so a thorough evaluation is required as any treatment strategy is employed.
Ultimately, the adoption of the hexavalent chromium MCL in California, the treatment strategies employed, and the experience gained will have long-lasting impacts on how other U.S. states and the world might institute future regulations of this metal for potable water sources. WW
References