Continuous Monitoring of Chlorine Helps Improve Plant Performance
Chlorine is used for disinfection, taste and odor control, and is a critical chemical in both the primary and secondary disinfection processes for drinking water. Continuous, on-line measurement of chlorine during disinfection is important because of the rising cost of chlorine and because of water quality concerns associated with both underfeeding and overfeeding of the disinfectant.
About 3000 KWH are required to produce a ton of chlorine from brine, and the delivered cost ranges from $160 to $300 per ton. As energy becomes more expensive, so inevitably will chlorine. In drinking water, underfeeding chlorine results in incomplete disinfection with consequent danger to the public health, while overfeeding chlorine produces water with an objectionable odor. Overfeeding also increases the levels in finished water of trihalomethanes (THMs), which are suspected carcinogens.
On-line chlorine monitoring systems typically look at free chlorine, total chlorine, or monochloramine, depending on system requirements. Although not an exhaustive explanation of the complexities of the chemistry of chlorine, here is a brief discussion of what happens when chlorine is added to water.
Water is chlorinated by treating it with chlorine gas (Cl2) or sodium hypochlorite (NaOCl) solution (bleach). When chlorine gas dissolves in water, it produces hypochlorous acid (HOCl).
Cl2 + H2O = HOCl + H + + Cl-(Equation 1)
Sodium hypochlorite solution is a source of hypochlorite ions (OCl-). Hypochlorous acid and hypochlorite ion are related to one another by the following equation:
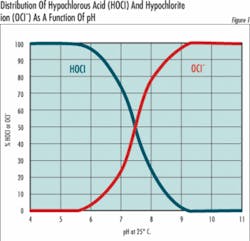
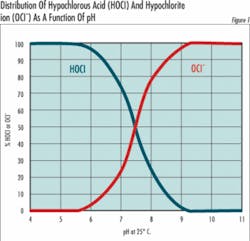
HOCl = H + + OCl- (Equation 2)The important thing about equation 2 is that any solution of chlorine gas or bleach in water is a mixture of hypochlorous acid and hypochlorite ions. The relative amount of hypochlorous acid or hypochlorite present depends on pH and to a slight extent on temperature.At 25°C (77°F) and a pH of 7.5, half of the chlorine is present as OCl- and half as HOCl. At higher pH values, the quantity of OCl- increases at the expense of HOCl and at lower pH values, the shift is toward conversion of OCl- to HOCl. At a pH of about 5, nearly all the chlorine is present as HOCl, and at pH 8.5 nearly all the chlorine is present as OCl-. Since the oxidizing power of HOCl and OCl- are very similar, the chemical and electrochemical methods used to determine chlorine concentration cannot distinguish between them, and it’s become traditional to express the two together as free residual chlorine (FRC):OCl- + HOCl = FRC (Equation 3)Why then all the fuss? Because the form in which the chlorine exists is very important when used in specific applications. For example, in disinfecting potable water, hypochlorous acid (HOCI) has been reported to be 80 to 100 times more effective than hypochlorite (OCI-). This means it takes 8 ppm of OCl- to do the same job of disinfecting as 0.1 ppm of HOCl.
Free Chlorine Measurement
Since the relative amounts of hypochlorous acid and hypochlorite ion are dependent on pH of the water, the reading from a free chlorine sensor typically depends on pH. Manufacturers of on-line chlorine measuring systems overcome the pH dependence by using reagents to adjust pH or by calculating a correction factor from the measured pH. Recently, a free chlorine sensor has been introduced that measures free chlorine independent of pH and does not require a pH sensor to make an accurate free chlorine measurement. Instead, the pH adjustment occurs inside the sensor, producing a reading that changes less than five percent per unit change in pH. This system can measure free chlorine in water having pH as high as 10.0.
Monochloramine
Although chlorine is an extremely effective disinfectant, in 1974 chlorination by-products were discovered in drinking water. Those by-products form when chlorine reacts and natural organic materials present in the water source. The by-products were found to have a potential health effect on humans and fears that they could potentially be carcinogens prompted the U.S. Environmental Protection Agency to establish maximum allowable levels for disinfection by-products.
As a result, alternates to chlorine disinfection have been developed. Monochloramine is one alternative. Although not as good a disinfectant as chlorine, monochloramine persists longer in the distribution system than chlorine, it produces negligible amounts of carcinogenic trihalomethanes and it contributes little or no taste or odor to the end-product water.
The process to produce monochloramine is called chloramination and involves adding chlorine and ammonia in the correct ratios to form monochloramine. Several chloramines can be made if the ratio varies, such as dichloramine and trichloramine. However, the most effective and important disinfectant is monochloramine. Plant operators using chloramination for disinfection need to accurately and continuously determine monochloramine levels throughout the water treatment and the distribution systems. Analysis equipment must directly measure monochloramine and for cost-effective operations, it’s best if analyzers do not require additional chemical reagents to condition the sample.
Total Chlorine
Total chlorine is the sum of free chlorine and combined chlorine. In other words:
total chlorine = free chlorine + combined chlorine or chloramines
More specifically, free chlorine consists of a mixture of hypochlorous acid and hypochlorite ion and chloramines can be a mixture of monochloramine, dichloramine and trichloramine. So it can be seen that the true definition of total chlorine is the sum of all five of the above chemicals. Drinking water treatment plants that use chloramination in secondary disinfection should only be making monochloramine. Therefore, they should only be concerned with measuring monochloramine and not total chlorine. If the correct ratios of chlorine and ammonia are added in secondary disinfection, an on-line measurement of total chlorine and monochloramine should be identical in concentration. This indicates that only monochloramine is being made, there is no free chlorine present, and no dichloramine or trichloramine exist.
Regardless of this, some municipal drinking water treatment plants want to measure the sum of free chlorine, monochloramine, dichloramine and trichloramine and the best way to achieve this measurement is by using a continuous, on-line analysis system for total chlorine.
About the Author
With over 20 years of industrial process and control experience, John Volbeda serves as industry manager for the water and wastewater industries for the Liquid Division of Emerson Process Management, Rosemount Analytical. Rosemount Analytical can be reached at www.raihome.com or Tel: 800-854-8257.