Disinfection Byproducts: Tewksbury WTP Pilots Aeration System, Online THM Monitor to Better Manage DBP Formation
By Lewis Zediana and William Clunie
The town of Tewksbury, Mass., services the needs of more than 30,000 residents through a 7 MGD conventional water treatment plant located on the banks of the Merrimack River, the primary water source for the Tewksbury Water Treatment Plant (WTP). The Merrimack is a dynamic body of water experiencing rapid changes in organic loading and ammonia concentrations due to effluent from the upstream Lowell Wastewater Treatment Plant (WWTP) and confluence of the Concord River.
While turbidity of the Merrimack River is typically below 10 Nephelometric Turbidity Units (NTU) 80% of the time, it has been measured as high as 1,540 NTU. Although ammonia levels are typically below 0.05 mg/L, a bypass event at the Lowell WWTP and water quality conditions during winter can lead to increased levels.
The Concord River, which makes up less than 10% of the total water volume for the Tewksbury WTP, is a slowing moving body of water containing high color and trihalomethane (THM) precursors.
The Tewksbury WTP currently uses chlorine dioxide and sodium hypochlorite addition for disinfection. Chlorine dioxide is generated onsite at the Tewksbury WTP using hydrochloric acid, sodium hypochlorite, and sodium chlorite. It is the primary disinfectant, while sodium hypochlorite is used to remove ammonia and maintain a residual and stabilize the water. Although disinfectant chemical treatment is dependent on influent raw water conditions, chlorine dioxide is typically dosed between 2 and 3 mg/L and sodium hypochlorite is dosed between 0.5 and 0.8 mg/L. Using a combination of chlorine dioxide and hypochlorite allows the Tewksbury WTP to address ammonia, taste and odor concerns while minimizing disinfection byproduct (DBP) formation.
THMs are the most significant DBPs generated and cause the greatest concern for the Tewksbury WTP since bromide levels are low in its source water and chlorinated DBPs are favored. Chloroform, one of the regulated THMs, dominates the town’s finished water as it has faster formation kinetics than other chlorinated DBPs.
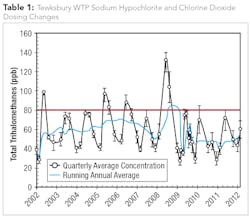
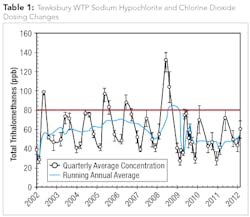
While the plant’s staff has been satisfied with the effectiveness of their current chemical disinfection and treatment process regime, plant operators are constantly monitoring the levels and subsequent impact on DBP formation resulting from the use of sodium hypochlorite and chlorine dioxide for oxidation and disinfection. For example, in 2009 the Tewksbury WTP experienced high levels of Total THMs (TTHMs) and, as a result, operators lowered the free chlorine dose while increasing the chlorine dioxide dose. The dosing changes were effective at reducing TTHM formation and highlighted the need for operational staff to explore additional process enhancements to further optimize treatment and minimize DBP formation (see Table1).
Disinfection Process Efficiencies
Following an engineering evaluation of the Tewksbury WTP completed by AECOM and issued in December 2012, additional disinfection process efficiencies could be realized through two modifications: First, an automated dosing control loop - currently under evaluation - to allow the plant to readily respond to rapidly changing raw water conditions and chlorine demands without promoting DBP formation; second, pilot test an aeration system for additional THM removal.
Because they are volatile organic compounds, THMs can be removed from water through volatilization given sufficient gas transfer opportunities. There are four primary species of THMs; chloroform (CHCl3), bromodichloromethane (CHCl2Br), dibromochloromethane (CHClBr2) and bromoform (CHBr3). Chloroform is the most volatile of the primary THMs and is the most prevalent THM speciation found in treated water at the Tewksbury WTP.
Packed towers, spray aeration, diffused aeration, and tray aeration are all methods of THM removal through air stripping. Each method has associated costs and gas transfer efficiencies. Air stripping using a combination of mixing and spray nozzles was the most applicable aeration approach to pilot at the Tewksbury WTP since the methodology is best applied in clearwells and/or distribution storage tanks.
The aeration pilot, managed by AECOM, was undertaken from September through November 2014. The three-month pilot program included a detailed study of the effectiveness of an air stripping aeration system at minimizing THM levels at the Tewksbury WTP. Influent and effluent THM values were measured for the duration of the study. In addition to traditional third-party laboratory analysis, an online THM monitor manufactured by Aqua Metrology Systems was also piloted for the duration of the study to measure THM values. Split sampling was used to determine how well the THM-100™ would compare to certified third-party laboratory analysis.
The validation of the THM-100 monitor found the instrument to be consistent and highly reproducible with a standard error of deviation of five percent. The automated online THM analyzer uses an approved “purge-and-trap” sampling method, followed by desorption into a chemical mixture that generates a colored product and time-resolved spectrophotometric analysis for detection and determination of THM levels. Manually collected “grab” samples from other locations can be analyzed alongside samples taken automatically by the monitor in its online mode, allowing for multi-point analysis.
The THM-100 monitor was installed near to the pilot plant and was used to analyze eight daily samples. The instrument provided plant operators with immediate results for Total THM and chloroform species measurements at the Tewksbury WTP. Prior to the use of the online THM monitor, plant operators waited to receive analytical results from external analysis, oftentimes conservatively operating the facility since real-time THM values were not known.
As a result of the real-time data provided by the online monitor, THM formation within the WTP and distribution system became easy to identify. The baseline and predictive data available through the THM-100 allowed process changes to be readily adapted to the current yet ever-changing water quality conditions at Tewksbury WTP.
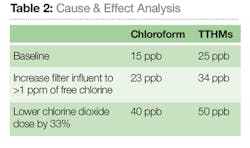
In October 2014, a cause and effect analysis was performed to see THM formation based on changes to the disinfection scheme (see Table 2). The analysis demonstrated the importance of controlling free chlorine in pre-treatment due to its dramatic effect on the production of TTHMs. Analysis further displayed the effectiveness of the various oxidants currently in use at the Tewksbury WTP to minimize THM formation.
With the conclusion of the aeration pilot study and successful operation of the online THM monitor, Tewksbury WTP is looking to install a THM-100 monitor in full-scale and permanent operation at the facility. The THM-100 will enable plant operators to have a firm and immediate understanding of THM values so they can better anticipate problems and maintain optimal treatment conditions at the Tewksbury WTP.
About the Authors: Lewis Zediana is the chief operating engineer for the Tewksbury (Mass.) Department of Public Works. William Clunie, P.E., BCEE, is a technical leader for water with AECOM.
More WaterWorld Current Issue Articles
More WaterWorld Archives Issue Articles