Water is one of the most vital resources for human life, yet about 2.2 billion people don’t have access to safe water and this water stress is expected to grow. As the world continues to tackle the global water crisis, researchers keep finding creative ways to disinfect drinking water and filter out contaminants. Many of the solutions listed below are very efficient, reusable, or low-cost — a major win for regions with low water security.
Inexpensive Hydrogels
A team from The University of Texas at Austin have created a hydrogel tablet that they say can rapidly neutralize bacteria in contaminated water. A single tablet may be able to disinfect a liter of river water, making it suitable for drinking within an hour.
“Our multifunctional hydrogel can make a big difference in mitigating global water scarcity because it is easy to use, highly efficient, and potentially scalable up to mass production,” Guihua Yu, an associate professor in the Cockrell School of Engineering, said in a press release.
The team also says that the process doesn’t require any energy input or create harmful byproducts, and can be easily removed without leaving any residue.
The team is working to improve the hydrogels by increasing the different types of pathogens and viruses in water that they can neutralize. The team is also in the process of commercializing several prototypes.
Scaling up the hydrogels’ production would be straightforward, the researchers say: materials for making them are inexpensive, and the synthesis processes remain simple at large scales.
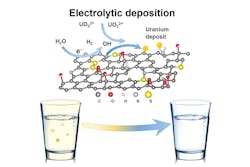
Metal-Filtering Foam
By applying an electric charge to graphene oxide foam, the researchers were able to capture uranium in solution in a condensed solid crystal. The team says that the foam may be reused up to seven times without losing its electrochemical properties.
“Within hours, our process can purify a large quantity of drinking water below the EPA limit for uranium,” Ju Li, the Battelle Energy Alliance Professor of Nuclear Science and Engineering, said in the press release.
The project began three years ago as an effort to find better ways to clean up heavy metals from mining sites. Earlier research had suggested that electrically charged carbon fiber might be able to filter uranium from water efficiently.
The team found that sending an electric charge through a graphene foam would split the water, releasing hydrogen and increasing the local pH. The higher pH helped induce a chemical change that pulled uranium ions out of a solution. The uranium would graft itself onto the foam’s surface, forming a never-before-seen crystalline uranium hydroxide, which resembles fish scales. Reversing the electric charge would let the mineral slip easily off of the foam.
The foam can capture four times its own weight of uranium, the team says. Coupled with an extraction capacity of 4,000 mg per gram, the foam’s reusability makes it an improvement over other methods.
The researchers have already begun investigating applying this method to other heavy metals, such as lead, mercury, or cadmium.
Solar-Powered Purification
According to a Princeton press release by Catherine Zandonella, the filter resembles a sponge and is able to soak up water while leaving contaminants behind. The purified water can then be easily collected from the sponge by placing it in sunlight.
The filter was inspired by the way that the pufferfish takes in water to swell its body when threatened and expel the water afterwards, Rodney Priestley, Princeton’s vice dean for innovation and co-inventor for the material, said in the press release.
“To me, the most exciting thing about this work is it can operate completely off-grid, at both large and small scales,” Priestley said. “It could also work in the developed world at sites where low-cost, non-powered water purification is needed.”
The filter employs a hydrogel that can either absorb or release water, depending on the temperature. The gel soaks up water at room temperature but pushes water out of its pores at 33° C (91 ° F).
The hydrogel is able to filter out petroleum, heavy metals, small molecules, and pathogens such as yeast. The team has also proven that the gel is reusable up to ten times without a drop in performance.
The team is now working to make the technology more widely available with help from Princeton Innovation, a program that helps Princeton’s researchers turn their discoveries into practical solutions.
Nanojars Catch Chromate
The nanojars are tiny containers made up of multiple repeating units of a copper ion, a pyrazole group, and a hydroxide. If the right ingredients are added to an organic solvent, the repeating units assemble into nanojars, with a –2 charged anion bound tightly at the center.
The researchers were able to remove anions from water by simply adding a solvent containing the nanojar components. The components formed an organic layer on top of the water.
To remove anions from water, the researchers added the solvent containing the nanojar components, which formed an organic layer on top of the water.
“The solvent doesn’t mix with the water, but the anions from the water can enter this organic layer,” explained Gellert Mezei, who presented the work via press release. “Then, the nanojars form and wrap around the ions, trapping them in the organic phase.”
The organic layer is then easily separated from the water. A weak acid can split the nanojars apart, releasing the anions for removal or recycling.
The team have successfully used nanojars to extract chromate and arsenate to well below the U.S. Environmental Protection Agency’s permitted levels for drinking water.
The researchers have also been working on making the process practical for real-world use. So far, all the experiments have been conducted at the laboratory scale. Developing a system to treat large volumes of water, such as in a lake, will require collaboration with engineers, Mezei said.
One day, contaminated lake water could be pumped into a station for treatment and then returned to the lake, the researchers claim. Some ions, such as phosphate, could then be recycled for useful purposes like fertilizer.
Reusable, Renewable Sorbent
The basis of the filter’s material is chitosan, a natural biopolymer obtained from chitin — one of the most abundant renewable materials on Earth. Chitosan has strong adsorption properties that easily form complexes with metal ions. The team’s new material is able to further enhance this adsorption.
“In a series of experiments, we found that the most productive processes of adsorption of copper and zinc ions proceed at room temperature and the pH of aqueous solutions equal to 3.5. The maximum, 60 percent adsorption of copper and zinc ions from aqueous solution, occurred within 40 minutes in the first case and 60 minutes in the second,” researcher Asmaa Abu El-Soad said in a press release. “Thus, our modified chitosan demonstrated better adsorption characteristics compared with such well-known sorbents as activated carbon, bentonite, shabasite, cellulose, rice husk, and others.”
The sorbent was also able to release the copper and zinc ions — suggesting that the technology is reusable and cost-effective.
Conclusion
The water sector is developing water treatment innovations at an impressive speed. Technologies like these will go a long way toward making a dent in the clean water crisis. WW
References
1. Guo, Y., et al. “Molecular Engineering of Hydrogels for Rapid Water Disinfection and Sustainable Solar Vapor Generation.” Adv. Mater. 2021, 33, 2102994. https://doi.org/10.1002/adma.202102994
2. Wang, C., et al. “Uranium In Situ Electrolytic Deposition with a Reusable Functional Graphene-Foam Electrode.” Adv. Mater. 2021, 33, 2102633. https://doi.org/10.1002/adma.202102633
3. Xu, X., Ozden, et al. “A Bioinspired Elastic Hydrogel for Solar-Driven Water Purification.” Adv. Mater. 2021, 33, 2007833. https://doi.org/10.1002/adma.202007833
4. Wisam A. Al Isawi, Matthias Zeller, and Gellert Mezei. “Capped Nanojars: Synthesis, Solution and Solid-State Characterization, and Atmospheric CO2 Sequestration by Selective Binding of Carbonate.” Inorganic Chemistry, August 27, 2021. http://doi.org/10.1021/acs.inorgchem.1c01826
5. Abu El-Soad, et al. 2022. “Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media.” International Journal of Molecular Sciences, February 21, 2022. https://doi.org/10.3390/ijms23042396
About the Author: Jeremy Wolfe is Assistant Editor for WaterWorld magazine. Email him at [email protected].
Published in WaterWorld magazine, May 2022.