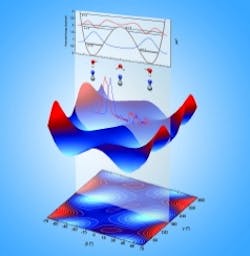
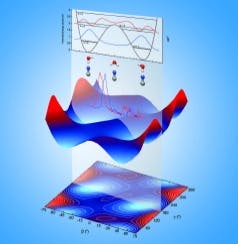
Hot and cold moves of cyanide and water: Temperature determines which molecule rocks out
RICHLAND, WA, Sept. 3, 2009 -- Scientists have long known that molecules dance about as the temperature rises, but now researchers know the exact steps that water takes with a certain molecule. Results with small, electrically charged cyanide ions and water molecules reveal that water zips around ions to a greater extent than expected. The findings improve our understanding of a chemical interaction important in environmental and atmospheric sciences.
"One of the cornerstones of Department of Energy nuclear cleanup missions and climate research is a fundamental understanding of water and ions, one of the most common chemical interactions in the environment," said chemist Xue-Bin Wang of the DOE's Pacific Northwest National Laboratory and Washington State University.
"We've developed a new instrument to probe the dynamics of ions in water," Wang said. "And we've combined theory and modeling to make sense of those experiments, giving us a deeper fundamental understanding of what is happening with this ubiquitous molecule -- water."
Wang, PNNL physical chemist Sotiris S. Xantheas, physical chemist Lai-Sheng Wang of PNNL and WSU, and their colleagues published the results in the Journal of Physical Chemistry A. The journal featured their work on the cover of its Sept. 3 issue.
For example, when common table salt -- sodium chloride -- dissolves in water, the negatively charged chloride ions (Cl -- ) and the positively charged sodium ions (Na+) each interact separately with the water molecules.
Previous work with chloride ions and water has yielded conflicting results about how a water molecule (which is shaped like a boomerang) and a chloride ion (shaped like a ball) face each other. Other groups study barbell-shaped cyanide ions because many molecules found naturally in the environment contain cyanide. The chemical interactions of water and either chloride or cyanide are influenced by the charge and the shape of the molecules, as well as the temperature in which they find themselves.
But directly observing temperature's role in how water and cyanide ions interact has been difficult. So, the team developed a unique instrument that allowed them to precisely control the temperature down to almost absolute zero, or the temperature at which everything freezes. The team used "temperature-controlled photoelectron spectroscopy" in EMSL, the DOE's Environmental Molecular Sciences Laboratory on the PNNL campus, to determine how tightly one cyanide ion and one to three water molecules interact at the very low temperature of -438 F (12 Kelvin) and again at ambient temperature of 80 F (equivalent to 300 Kelvin).
Unexplained Energy
The team measured the molecules' "electron binding energy" at low and high temperatures. This energy is an indication of how tightly the molecules hold onto their electrons -- the tighter the hold, the stronger the bonds that will form between molecules. The team found that ones at low temperature exhibited higher electron binding energy than the ones at high temperatures, as they had expected. However, the difference between the two scenarios was greater than the team expected.
The team found that the molecules behaved differently at cold and warm temperatures. At lower temperatures, the boomerang-shaped water held still while the cyanide teetered at the end of one of water's two arms. There, the cyanide flipped, sometimes pointing its carbon (C) atom towards the water's arm, and sometimes pointing its nitrogen (N). At the coldest temperature tested, -438 F, the molecules froze, with cyanide pointing its nitrogen end at the water.
Hot to Trot
At ambient temperatures, however, the barbell-shaped cyanide held steady while the water molecule rocked and flipped around the cyanide. Although the researchers were surprised at how much the water moved, the many positions water could take explained why they saw less electron binding energy than they expected at room temperature: A wiggly water means that the bond between molecules isn't that tight.
"Water can interact with cyanide's carbon or nitrogen and rock back and forth on one atom," said Wang. He added that the detail they get with this instrument is impressive. "Scientists have known for years that atoms move around when temperature rises. Now they can determine the most probable position that the molecule is in at different temperatures."
The results also explain the conflicting results with chloride ions and water, the researchers said, because of the importance of temperature on that interaction as well.
The researchers plan to follow up with studies that include many water molecules and ions at once, as well as with more complex ions than cyanide.
Other authors include PNNL's Karol Kowalski and Alfred Laubereau and Jasper Werhahn from the Technical University of Munich at Garching.
Reference: Xue-Bin Wang, Jasper C. Werhahn, Lai-Sheng Wang, Karol Kowalski, Alfred Laubereau, and Sotiris S. Xantheas, Observation of a Remarkable Temperature Effect in the Hydrogen Bonding Structure and Dynamics of the CN -- (H2O) Cluster, J. Phys. Chem. A, DOI 10.1021/jp9034002 (http://pubs.acs.org/doi/abs/10.1021/jp9034002
This work was supported by the Department of Energy's Office of Basic Energy Sciences within the Office of Science.
EMSL, the Environmental Molecular Sciences Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science, Biological and Environmental Research program that is located at Pacific Northwest National Laboratory. EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. EMSL's technical experts and suite of custom and advanced instruments are unmatched. Its integrated computational and experimental capabilities enable researchers to realize fundamental scientific insights and create new technologies. EMSL's Facebook page
Pacific Northwest National Laboratory is a Department of Energy Office of Science national laboratory where interdisciplinary teams advance science and technology and deliver solutions to America's most intractable problems in energy, national security and the environment. PNNL employs 4,250 staff, has a $918 million annual budget, and has been managed by Ohio-based Battelle since the lab's inception in 1965. Follow PNNL on Facebook, Linked In and Twitter.
###