Project Seeks to Balance Corrosion Control, Scale Formation
by Tim Brodeur and Ryan Florence
The Tampa Water Department (TWD) has been engaged in a series of studies examining the control of Calcium Carbonate Precipitation Potential (CCPP) with the goal of both controlling corrosion in the distribution system while also preventing excess calcium precipitation within the distribution network.
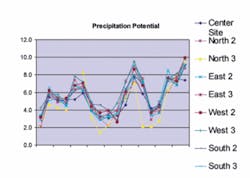
System-Wide CCPP Monitoring Preformed by TWD's Lab
The latest study in the series has led to the development of computerized modeling and planning tools that have facilitated the complex analysis and calculation scheme to allow better interactive control of corrosion/scale formation and to allow more precise operational control through the use of real-time information.
Background
Tampa operates a surface water treatment system that uses standard and Actiflo coagulation/sedimentation processes to reduce color and Total Organic Carbon (TOC), ozonation for oxidation and primary disinfection, biological enhanced filtration, followed by chlorine/chloramine for secondary disinfection. The raw water source for the plant is a reservoir on the Hillsborough River adjacent to the plant.
A lack of rainfall in the Hillsborough River watershed in the late 1990s prompted the Tampa Department to begin supplementing its water supplies with groundwater from Sulphur Springs. The groundwater source, however, contained high total dissolved solids (TDS) in the form of calcium hardness and chlorides and raised the TDS concentrations of the reservoir source. The higher calcium hardness in the blended raw water required operations to adjust the plant’s process chemistry to prevent precipitation of calcium on the equipment.
These changes resulted in the finished water chemistry altering the tuberculation/corrosion layer in the matrix of distribution system piping, leading to a series of “red water” complaints from customers.
To counter the problem staff raised the pH after the sedimentation process by chemical addition (mainly lime and sodium hydroxide). This, in turn, led to issues with high service pumps and meter failures due to rapid precipitation of calcium carbonate. A subsequent investigation into water losses within the system revealed that venturi meters located inside the plant and service meters outside the plant contained calcium carbonate coatings that likely reduced metering accuracy.
In this most recent study, Boyle Engineering examined the facility operating data, TWD flow meter studies and developed computerized modeling/planning tools. The computerized tools use Visual Basic for Applications (VBA) within a Microsoft Access database to facilitate the determination of CCPP on a large set of data and to tie the calculations to the facility’s Supervisory Control and Data Acquisition (SCADA) data tables.
The current approach through modeling and continuing operational data collection is to reduce the CCPP to a range of 2-5 mg/L, maintain a tighter deadband on either side of the range and optimize chemical costs.
Use of CCPP In Corrosion Control
CCPP is an accurate, versatile measure of the potential for a water to deposit or dissolve CaCO3; however, it was never intended to serve directly as a corrosion index. Although deposition of a protective film of CaCO3 has been thought to provide a sound method of corrosion control since the early 1900s, research shows that CaCO3 saturation indexes may not provide an accurate indication of corrosion control ability.
While the CCPP value indicates the potential for CaCO3 to precipitate or dissolve, it does not indicate when or where precipitation or dissolution will occur. Historical analysis of the Tampa Water Department facility operation shows that as the CCPP control parameter was adjusted, areas where excessive scaling occurred also changed.
Also, CCPP may indicate the quantity of CaCO3 deposited, but it does not indicate the quality of the CaCO3 film or its ability to provide protection against corrosion. The CaCO3 film may not achieve the required density, uniformity, or adherence to the pipe.
Establishing Operational Finished Water CCPP Ranges
Although CCPP should not be the sole corrosion-control criteria, when used in conjunction with other control parameters it is a valuable control index. In the case of the Tampa Water Department, the value of CCPP for maintenance of the protective CaCO3 coating is underscored through correlation with the department’s historical distribution system performance.
As part of establishing a corrosion control strategy through use of CaCO3 passivation, it is first necessary to answer the question of how much CaCO3 precipitation is appropriate for a given distribution system. Since the inception of the Langelier Index, the conventional wisdom has been to maintain CaCO3 in a slightly oversaturated state. With the introduction of CCPP, the range of 4-10 mg/L has been considered desirable.
However, the “right” CCPP range will not be the same for all systems; it is best decided through correlation with distribution system performance. In Tampa’s case, when the department adopted the 4-10 mg/L operating range for its finished water, failures of the high service pumps were experienced due to excessive CaCO3 scaling.
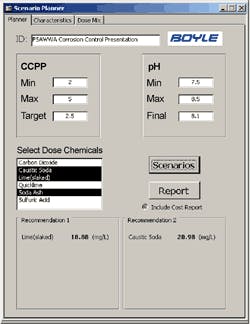
CCPP and pH Input Parameter Window with two dose recommendations
Subsequently, Tampa lowered its operating CCPP range to 3-7 mg/L with an ideal target set at 5 mg/L. The excessive scaling seemed to “move away” from the plant but then increased in the distribution system, resulting in residential meter inaccuracies.
This latest study recommended to the Tampa Water Department an adjusted CCPP range of 2-5 mg/L, which would offer the necessary balance between pipe corrosion and excessive CaCO3 deposit. Although the CCPP range recommendation was based in part on historical data, additional considerations also suggest the new CCPP range.
First, the lower operational boundary of 2 mg/L is considered reasonable because it maintains the desired oversaturation of CaCO3. Theoretically, a CCPP value that is slightly greater than zero would be close to the CaCO3 saturation at equilibrium and therefore desirable. However, a limit less than 2 mg/L could lead to occasions where water leaves the plant dissolving CaCO3 scale, disrupting the protective coating, which could lead to another red water episode. Therefore, 2 mg/L provides a reasonable factor of safety against weakening the protective coating, yet remains close to the CaCO3 equilibrium saturation.
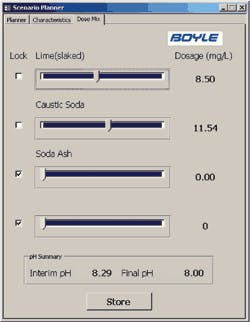
Example of Dose Mix Feature of CCPP Scenario/Planner Tool
Second, a 3 mg/L operational range or “window” was established by answering the question, “How tight of a CCPP control range can reasonably be required and maintained?” With proper plant monitoring mechanisms in place and rapid water quality analysis it may be possible to achieve a smaller spread in CCPP operating parameters; however, a control range less than 3 mg/L could not be justified at this point.
CCPP Control Mechanism
With the operational CCPP range established, it was critical that the appropriate control mechanism was in place at the plant to achieve the desired finished water condition. Therefore, Boyle worked with Tampa to develop the computerized modeling/planning tools to aid in finished water CCPP control and scenario planning.
Prior to the project, a pH window was used as the primary CCPP control mechanism. However, using a pH range or window to control CCPP poses some difficulties. Generally, as pH is decreased the precipitation potential of CaCO3 decreases; inversely, the CCPP is increased as pH is increased. Depending on the treatment chemical selected, however, change in pH does not equally affect change in CCPP.
As shown in Table 1, achieving a pH goal may produce a variety of different outcomes for CCPP, depending on the treatment chemicals selected; therefore, CCPP control is best achieved by considering treatment alternatives against outcome on CCPP while satisfying pH criteria.
With SCADA based input data, the scenario planning tool developed during the Tampa project is aimed at generating viable treatment alternatives that meet CCPP finished water goals while economically utilizing treatment chemicals. The system is generally based on methods established by Rossum and Merrill, and the results have been verified against the Rothberg, Tamburini & Winsor (RTW) “Model for Corrosion Control and Process Chemistry.”
The Boyle tool allows the operator to input water characteristics (either manually or from the SCADA data tables), input CCPP and pH parameters, and quickly evaluate treatment alternatives. The tool also allows the operator to consider chemical mixture alternatives with the “Dose Mix” feature of the tool and a sample of the output data.
Although the tool provides a direct and simple way for an operator to evaluate treatment alternatives, limitations in maintaining the suggested CCPP range due to raw water conditions will still present challenges in corrosion control. The primary aim, however, is to provide a means of better controlling CCPP to reduce the large variations in CCPP evident historically and to aid in maintaining an effective CaCO3 coating on pipe walls.
About the Authors:
Tim Brodeur, PE, is a Principal Engineer with Boyle since 2002, where he specializes in Environmental Engineering projects. As the lead for Boyle’s water programs and practices in Florida, Brodeur’s 35 years of expertise includes directing the firm’s water process group in water supply planning, process engineering, treatment procedures and design for municipal water supply projects. Ryan Florence has been an Assistant Engineer with Boyle since 2003. As Boyle’s lead programmer for the Tampa Water Department project, Florence specialized in database programming and algorithm development for chemical analysis. Also contributing to this project were F. Scott Davis of Boyle Engineering and Myung Kim, PhD; Marjorie Craig, PE; Jim Gianatasio; Dawn Sharp, MPH; and Paula Lowe, all of the City of Tampa.