Product Focus: Video Explains Chlorination, Chloramination Curve
As the Stage 2 Disinfectant/Disinfection Byproducts Rule deadline approaches for many drinking water utilities, the balancing act of compliance continues to be a challenge. Utilities must insure they are meeting disinfectant residual requirements, but also must be careful not to create excess byproducts. Whether a utility is using chloramines or free chlorine as their disinfection residual, the margin for error in dosing and distribution system control is becoming smaller.
In today's environment of tighter control and monitoring, it is important to understand the levels of different chlorine and ammonia species in the water. Many chloraminating utilities have traditionally relied on a dosing ratio to control their process, such as five parts chlorine to one part ammonia. The dosing ratio is a starting point, but does not account for effects of varying pH levels, temperature, or existing ammonia in the water and chlorine demand in the source water.
Utilities that are purchasing water are especially challenged by these factors. For example, if the disinfectant demand in source water increases during the summer as water temperatures rise, water treated with dosing levels that were set correctly earlier in the year may leave the plant with excessive levels of free ammonia, which is proven to lead to nitrification issues.
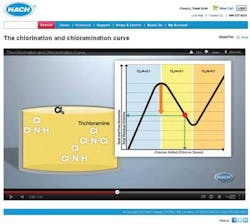
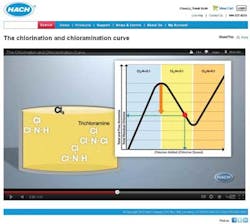
Chlorination and Chloramination Curve
To help utilities understand where their chlorine and ammonia levels lie, and how to make adjustments to reach optimum disinfectant levels, Hach Company has created an animated educational video titled, "The Chlorination and Chloramination Curve." The video discusses the chemistry in terms that are easy to understand and helps demystify the science behind water disinfection. The video is free to watch online at www.hach.com/animation.
The video describes exactly what's happening with disinfectant residuals, and how to know if you are at risk of nitrification (which can result from a high ammonia to chlorine ratio) or, on the other side of the chloramination curve, with too much chlorine. The following observations from the video may be particularly useful:
1. You may not really know where on the chlorine curve your utility's water lies unless you test. If you measure for total chlorine, monochloramine, free chlorine, free ammonia, and total ammonia, it is easy to know where on the curve your utility's water is at any given time.
2. With oversaturation of chlorine, you can run into taste and odor issues, as well as a drop in disinfecting power.
3. The measurement of free ammonia is critical to understanding if you are maximizing monochloramine production and reducing risk of nitrification.
Dosing Method Improvements
"Utility operators may overlook a measurement that reveals a drop in chlorine residual as chlorine is added, because it is counterintuitive. But, if the balance is not corrected, chlorine residuals may remain too low to sustain water quality through the distribution system," said Gary Visser, Regional Sales Manager at Hach.
On the other side of the chloramination curve, if a utility doses too much ammonia or not enough chlorine, free ammonia may be present in the distribution system. This may lead to nitrification problems, including water quality, health, and regulatory compliance issues. If nitrification is left uncontrolled, costly line flushes may be required. The biggest problem for many utilities is a reduction in total chlorine residuals. Nitrification can present additional issues, and can also lead to lower alkalinity and pH levels, which can increase distribution system corrosion.
Visser notes that additional tests can be useful for proactively maintaining water chemistry levels.
"Hach recommends testing monochloramine, free ammonia, and total ammonia in addition to chlorine for chloraminating systems. Instruments to perform these tests are readily available, and may save utilities tens or even hundreds of thousands of dollars in potential flushing and labor costs, or lead to reductions in chemical use (due to more accurately managing the chemical balance)," he said. "This additional testing is not hard to do and, more importantly, it can make a big difference in water quality and problem aversion. Utility operators can think of it as water quality insurance that, like other types of insurance, provides peace of mind."
Another common oversight for chloraminating systems is the misconception that you can put the widely accepted ratio of 5:1 chlorine and ammonia into the water and expect the ratio to remain stable. Visser notes that the ammonia to chlorine ratio is a great tool for getting close to the right measurement, but may not be enough to ensure a maximized monochloramine concentration to total chlorine.
"Just having that feed ratio is a lot like getting directions from your town to the state capital that tell you: 'Get on I-95, go north for two hours, and you'll be at the state capital.' Those directions will get you close to the capital, but what you really need is a big sign on the interstate saying, 'Capital, exit here.' That 'sign' in terms of water testing is free ammonia. When we find the free ammonia break-point, we have reached our destination," Visser said.