New Technology Detects Large Concentrations of Hydrogen Sulfide in Beer and Wine
Summer is here. And what activity better exemplifies summer than enjoying an ice cold beer? However, if that beer smells faintly of rotten eggs or has a dreaded "skunky" flavor, it can ruin an otherwise idyllic afternoon.
What causes these undesirable flavors in beer? During fermentation, the yeast which converts simple sugars into alcohol also naturally produces some hydrogen sulfide (H2S). Low levels of H2S are actually desirable and give the beer complex, defining flavor characteristics. However, at higher concentrations, H2S is responsible for the malodorous smell, and the interaction of H2S with the hops used in the brewing process is responsible for the unpleasant aroma in bad beer. Excess H2S can be a symptom of unhealthy yeast, microbial infection or improper oxygen levels during fermentation, as well as myriad other root causes.
Due to the volatility of H2S (b.p. = -60°C), one effective method for testing the concentration of dissolved H2S is to test the headspace above the liquid. If the temperature and accumulation time are well controlled, then the concentration of H2S in the headspace will be proportional to the concentration of H2S dissolved in the sample. Brewers have sophisticated sensors in their fermentation tanks to monitor the H2S concentration during production, but as anyone who has bought an "off" case of beer knows, bad beer can sometimes make it into the bottle.
Arizona Instrument LLC has a solution for the determination of H2S in bottled beer using the Jerome® J605 Hydrogen Sulfide Analyzer. The method can be processed in under seven minutes and can be used to determine H2S concentration in beer as low as 5 parts per billion (ppb). No hazardous materials are required for testing, and the instrument response over the range investigated was linear with respect to concentration.
To run a test, an Erlenmeyer vacuum flask is connected to a Jerome® J605 Hydrogen Sulfide Analyzer by a tygon or other suitably-sized inert tubing. A full bottle of beer is poured into an Erlenmeyer flask and allowed to stir for five minutes. The instrument is placed in an auto-range and auto-sample, testing the headspace above the beer every two minutes. Further, the instrument is allowed to sample for 30 minutes, and the results are then summed.
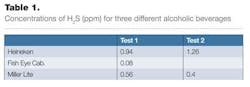
From Table 1, we can see that the Heineken has the highest concentration of H2S. This should be what is expected, since it also has the strongest flavor of the tested beverages. The wine has the lowest concentration, which is also expected, since wine gets its flavor from other sources.
Arizona Instrument LLC is an exhibitor at the WEFTEC.13 event and can be found at Booth No. 1005.