CORROSION CONTROL: Using Caustic Soda to Control Corrosion and Calcium Carbonate Precipitation
By Ferdous Mahmood, Bruce Utne and Patricia Gamby
The decay of distribution system infrastructure due to corrosion is a major challenge facing water utilities in the 21st century. In addition to red water problems which commonly result from the corrosion of unlined cast iron distribution pipes, there are numerous other problems associated with pipeline corrosion that affect water quality and the cost of operating and maintaining a distribution system.
Calcum carbonate precipitate in drinking water clearwell.
Generally, the goal of corrosion control strategies is not only to reduce the corrosion of distribution mains but also to minimize the release of lead and copper from residential plumbing fixtures. Lead and copper are regulated under the Lead and Copper Rule of the Safe Drinking Water Act and in many cases determine the corrosion control treatment technique employed by utilities. Two commonly used treatment techniques for controlling the release of lead and copper include lowering the corrosivity of water with pH adjustment and/or with the addition of a corrosion inhibitor.
Increasing the pH decreases the solubility of lead and copper in water and may lead to the formation of an insoluble calcium carbonate precipitate along the pipe wall. The deposition of calcium carbonate in pipes may help reduce corrosion by providing a barrier on the pipe wall which prevents water from coming into contact with and corroding the pipe material. Similarly, corrosion inhibitors can reduce the corrosion of distribution system pipes and prevent lead and copper dissolution by forming an insoluble coating of metallic orthophosphate. Utilities often employ pH adjustment as a corrosion control strategy because treatment plants already have chemical feed systems.
For waters with moderate to high levels of hardness and alkalinity, increasing the pH of the finished water may also promote the precipitation of calcium carbonate within the distribution system. Excessive deposition, however, will significantly reduce the hydraulic carrying capacity of the pipes. This problem is particularly true for utilities that use lime for post treatment pH adjustment.
Lime adds calcium ions and increases the pH which shifts the distribution of carbonate alkalinity from bicarbonate (HCO3-1) to carbonate (CO3-2) alkalinity. When the concentrations of calcium and carbonate ions exceed the solubility product, calcium carbonate precipitate is formed. Therefore, if lime is added indiscriminately, the result can be excessive amounts of calcium carbonate precipitation in post treatment storage facilities or within the distribution system.
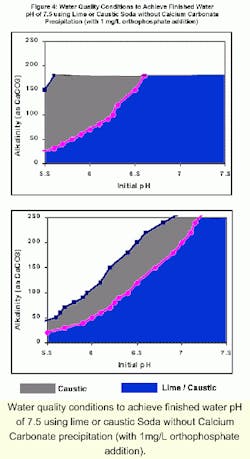
Water quality conditions to achieve finished water pH of 7.5 using lime or caustic Soda without Calcium Carbonate precipitation (with 1mg/L orthophosphate addition).
Experience has shown that it is difficult to develop a uniform deposition of calcium carbonate throughout the distribution system. Deposition generally occurs close to the treatment plant and less protection is provided as the water travels further from the plant, typically where the lead and copper containing fixtures are located. To prevent reduction in hydraulic carrying capacity of pipes, corrosion control strategies have generally evolved into producing a low corrosivity water with a low scale forming potential. As a guideline, water leaving the treatment facility should have a calcium carbonate precipitation potential (CCPP) greater than 0 but less than 10 ppm to provide protection without contributing to the excessive deposition within the distribution system.
One possible means of reducing the corrosivity of water while minimizing the excess deposition of calcium carbonate is to switch post treatment chemicals for pH adjustment from lime to caustic soda. Caustic soda, or sodium hydroxide (NaOH), will increase the pH of the water without adding calcium ions that are needed for calcium carbonate precipitation to occur. Consequently, for a given pH adjustment, caustic soda should reduce the calcium carbonate precipitation potential compared to lime.
In some cases, however, there is sufficient calcium already present in the water that caustic soda may not provide any significant benefit over lime in terms of reducing the calcium carbonate precipitation potential of the finished water. In addition to the background calcium concentration, the initial pH, desired finished water pH, alkalinity, and temperature also play significant roles in determining if caustic soda will be able to reduce the CCPP of the water compared to lime.
Because of the many factors affecting calcium carbonate precipitation, an analysis covering a wide range of water quality conditions should be performed to determine when caustic soda may provide a benefit over lime in terms of controlling corrosion and reducing the precipitation of calcium carbonate in finished water reservoirs and distribution systems.
Case Study
An analysis was performed for the Washington Aqueduct which provides water to Washington, D.C., and northern Virginia. The Washington Aqueduct operates two surface water treatment facilities with a combined treatment capacity of 315 mgd. The Dalecarlia water treatment plant, which is the larger of the two facilities, provides conventional treatment consisting of pre-sedimentation, rapid mix, flocculation, sedimentation, and media filtration. Currently, lime is added for finished water pH adjustment after filtration and prior to distribution. The finished water pH generally ranges between 7.5 and 8.5.
The Rothberg, Tamburini and Winsor (RTW) Model for Corrosion Control and Process Chemistry (AWWA, 1996) was used to evaluate and compare the use of caustic soda and lime for finished water pH adjustment. The RTW model uses equilibrium chemistry to predict water quality parameters and has been widely used to evaluate the corrosion potential of water in distribution systems. The model calculates two commonly used "corrosion indices," the calcium carbonate precipitation potential (CCPP) and the Langelier saturation index (LSI). Both indices provide a measure of the scale forming potential of the water.
The RTW model was used to calculate the CCPP of the finished water using historical water quality and chemical use data at the Dalecarlia WTP. As would be expected from the large amounts of calcium carbonate precipitate that accumulated in the finished water reservoir, the RTW model predicted that significant calcium carbonate deposition would occur under the current operating conditions. The model also indicated that the CCPP varied throughout the year with higher CCPP coinciding with the warmer months of the year when calcium carbonate was less soluble. The pH of the finished water varied between 7 and 8 depending on the season of the year.
A consistent finished water pH of 8.5 was desirable to reduce the corrosivity of the water if corrosion inhibitors were not used. The RTW model was then used to calculate the CCPP which would have occurred if the finished water pH was maintained at 8.5 using lime or caustic soda for the same historical period. The study found that caustic soda would reduce the CCPP compared to lime. However, for a finished water pH of 8.5, the CCPP was still greater than the target range of less than 10 mg/L CaCO3 for most of the year for both chemicals. This result is attributed to the fact that the filtered water contained sufficient amount of calcium hardness and bicarbonate alkalinity (approximately 80 mg/L and 50 mg/L as CaCO3, respectively) for precipitation of calcium carbonate. Consequently, for the Washington Aqueduct, caustic soda would not provide a significant benefit over lime in terms of reducing the amount of calcium carbonate deposition in the finished water reservoir and in the distribution system.
Although caustic soda would not provide much benefit for the Washington Aqueduct, there are some water quality conditions where caustic could provide some benefit over lime. The figures below show the results of RTW model runs at 25
The shaded regions in in the figure represent the water quality conditions for which the respective chemicals could be used without precipitation of calcium carbonate (CCPP = 0 mg/L). The blue region denotes the water quality conditions where both lime and caustic soda could be used to achieve a finished water pH of 7.5 without precipitation of calcium carbonate. The gray region denotes the water quality conditions where only caustic soda could be used for pH adjustment, and the use of lime would produce excessive precipitation.
For example, when the calcium hardness is 30 mg/L as CaCO3, lime can be used to increase the pH from 6 to 7.5 without excessive precipitation only if the alkalinity is less than 70 mg/L. Caustic soda must be used to prevent excessive precipitation for higher alkalinity waters (in excess of 200 mg/L as CaCO3). When the calcium hardness is 90 mg/L as CaCO3, lime can be used to increase the pH from 6 to 7.5 without excessive precipitation only if the alkalinity is less than 50 mg/L. Caustic soda must be used to prevent excessive precipitation for higher alkalinity waters but only if the alkalinity is less than 110 mg/L. These results are applicable at 25 0C. For lower temperatures, a wider range of water quality conditions can be used for pH adjustment with lime and caustic soda.
Conclusion
The decay of distribution system pipes is a major challenge to water utilities in the 21st Century. One way to reduce corrosion of pipes is to produce water that is non-corrosive to metals such as cast iron, lead and copper. Non-corrosive water will not only prolong the life of pipes but also reduce lead and copper levels in the water. One way to reduce the corrosivity of the water is to increase the pH. This increase can be easily achieved at the treatment plant where chemicals and feed systems are already available for pH adjustment.
For some waters, increasing the pH may lead to excessive precipitation of calcium carbonate. This precipitation can result in excessive deposition of the precipitate in finished water clearwells and decreased hydraulic capacity of pipes.
The use of caustic soda instead of lime may reduce calcium carbonate precipitation while achieving the desired finished water pH. However, the benefit of using caustic soda over lime can be realized only under certain water quality conditions. Generally, caustic soda provides some benefit over lime for soft waters. The benefit of caustic over lime decreases as the calcium hardness increases.
About the Authors: Ferdous Mahmood is a Project Engineer at Malcolm Pirnie, Inc. Bruce Utne is Water Production Manager at Newport News Waterworks. Patricia Gamby is Chief of Waterworks/Environmental/ Electrical at the Washington Aqueduct Division of the U.S. Army Corps of Engineers.