Streaming Current Monitor Used to Optimize Coagulant Dosages
By Linda Kramer and John Horger
The city of Philadelphia operates three water treatment facilities which serve a population base of approximately 1.5 million citizens. All three of the plants operate using the conventional treatment method consisting of chemical pre-treatment, flocculation/ sedimentation, filtration and disinfection.
The Samuel S. Baxter Water Treatment Plant is the largest drinking water facility in the city of Philadelphia, with an annual average finished water effluent of 175 mgd. The Philadelphia Water Department (PWD) has been a member of the Partnership for Safe Water since 1996. It is one of over 200 utilities nationwide which have agreed to adhere to more stringent water treatment goals to improve the quality of their drinking water. Since joining this partnership, PWD has sought to optimize the water treatment process and thereby improve water quality.
In 1998, the plant began a half-plant trial to achieve coagulant optimization and improved TOC removal while maintaining finished water turbidities below 0.10 ntu. The success of this trial enabled the plant to achieve Stage 1 Interim Enhanced Surface Water Treatment Rule (IESWTR) TOC removal goals while operating in enhanced coagulation. Full-scale plant conversion to enhanced coagulation with dual pH adjustment has just begun at the facility.
Historical Treatment
Water is drawn from the Delaware River twice daily as the tide flows upriver. The river is a stable raw water source with a low average incoming turbidity of 6.0 ntu. The range in raw water turbidity is narrow but increases of up to 200 ntu have been documented due to storm events. More typical storm water turbidity ranges are from 20 to 40 ntu. The plant has a 176 million gallon raw water storage facility which is seasonally dosed with a preoxidant, potassium permanganate, to control algae and associated tastes and odors. The plant is a captive facility whose incoming flow rate is determined by an off-site distribution unit, which monitors water storage and usage levels throughout the city. Peak flow throughout the plant is approximately 282 MGD.
Baxter personnel continue to look at many tools to optimize their treatment process while keeping a watchful eye on the cost of drinking water to the consumer. The streaming current monitor (SCM) is one such on-line instrument that can be helpful in optimizing the coagulant dosage. The Baxter WTP uses ferric chloride as its coagulant.
The Baxter Plant purchased an SCM to monitor the effectiveness of its coagulant dose on a real time basis. The usefulness of the monitor under both sweep flocculation and charge neutralization was examined at the Baxter WTP. Throughout the half-plant enhanced coagulation trial, the north side of the Baxter WTP coagulated using sweep flocculation while the south side coagulated using charge neutralization. Streaming current monitors were placed in the process stream on both the north and south sides of the plant to study their ability to optimize coagulant dosages.
Theory of SCM Operation
The SCM operates by using an electronic sensor to determine whether charge neutralization has occurred after the addition of coagulant to the raw water. The addition of ferric chloride adds a positive charge to the raw water from the Fe+++ ion. If the correct coagulant dosage is added to the incoming raw water it should exactly neutralize the negatively charged particles in the raw water. In theory, if you have the proper coagulant dose and a complete mixing of the chemical has occurred, the SCM will read "0.0".
The SCM uses a zeta potential charge-measuring device to measure the net ionic and colloidal surface (positive and negative) charge in the water. As water is moved back and forth by the piston, charges surrounding these particles (+ and -) are moved downstream to the electrodes, thus producing a streaming current. The signal detected by the electrodes is then amplified. The streaming current amplitude and polarity is a function of sampling location and the type of coagulant being used in the treatment process.
Summary of Results
The North Side treatment train operates in the non-enhanced coagulation mode. This side of the facility has only one pH adjustment point at pre-treatment. To obtain a finished water pH of 7.4, the coagulation process must occur at a pH range of 8.0 - 9.2 depending on raw water alkalinity. Under these operating conditions it is impossible to coagulate using charge neutralization. Therefore sweep flocculation occurs.
The SCM proved unresponsive at the Baxter Plant during high pH coagulation. The unit's sensor quickly became coated with lime, which further hindered its ability to respond to the change in water chemistry conditions. The SCM would move in a positive direction when the ferric chloride dosage was raised to 150 percent of the normal plant dosage. It would likewise move in the negative direction when the normal plant dosage was decreased by 50 percent. The unit was also tested at the highest gain setting, which is the most sensitive to changes in the water sample.
The typical plant coagulant dosage change at the Baxter WTP is 10 - 20 lb/MG which is generally 5-7 percent of the total coagulant dosage. The SCM would not respond to these small dosage changes. After numerous attempts to relocate and successfully operate the SCM under these treatment conditions the SCM was shut down.
In 1998, the Baxter WTP began a half plant trial on the south side of its treatment process to look at enhanced coagulation with a dual pH adjustment. The goal of this trial was to improve TOC removal, reduce formation of disinfection byproducts and optimize coagulation. The pH of coagulation was then lowered to a new goal of 6.5. This lower pH of coagulation coupled with the installation of a SCADA monitoring system at the Baxter Plant renewed interest in the SCM as a tool to optimize coagulant dosage.
The sample location at the Baxter WTP is located in the rapid mix area. This location is approximately five to 10 minutes after the addition of ferric chloride and two minutes after the addition of hydrated lime. Jar testing has shown that this is an ideal point for charge neutralization to have reached completion.
A key component of SCM operation involves the calibration of the instrument. The streaming current monitor should be cleaned and calibrated using a standard operating procedure. This procedure should remain the same under all operating conditions to provide the operator with a standard zero setpoint from which to interpret the SCM trends. At the Baxter WTP staff chose a calibration method which uses the pH 7 neutral stock solution as the zero setpoint calibration sample. The Baxter SCM is cleaned weekly to remove coatings from the sample cell and sensor.
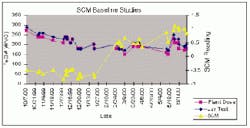
Baseline Studies
Tests were performed to establish the range of SCM readings when the plant was operating at its optimal coagulant dosage. These tests were performed over all four seasons to record the effects of seasonal water quality changes. Jar tests were performed to determine the optimal plant ferric chloride dosage and then record the corresponding reading of the SCM.
Baxter baseline studies indicate that for the fall and winter months the baseline value for the SCM ranges between -0.40 and -0.60. Once the Delaware River is influenced by algae and throughout the summer months the baseline then becomes positive in the range of +0.50 to + 0.80.
Baxter personnel now routinely perform jar tests to compare the optimal coagulant dosage as a method to confirm the baseline SCM reading. The ability to periodically confirm the baseline SCM reading helps to validate the reliability of the instrument. The baseline database and seasonal trends recorded in the SCADA will be able to provide Baxter personnel with a historical reference to interpret future SCM trends.
Successful Uses
Baxter chemists and engineers have successfully used the SCM for coagulant optimization, storm events and feed equipment failures. The streaming current monitor responds well to changing raw water conditions and enables the process control chemists to trim coagulant dosages. The chemists use conventional water quality indicators such as raw water turbidity, combined filter effluent turbidity and visual inspection of the flocculation and sedimentation basins as their primary standard for establishing their coagulant dosage. The SCM becomes a useful tool in trimming or adding the extra 10 - 20 lb/MG of coagulant to optimize the dosage. This can lead to cost savings as well as increased TOC removal.
On September 16, 1999, Hurricane Floyd struck the Southeastern Pennsylvania region with extreme rains and heavy flooding. The Baxter raw water intakes began to see the effects of the storm for the next four days. Raw water turbidity rose to 46 ntu and remained high for a 5-day period ranging from 46 to 28 ntu. Windswept heavy rains and floating debris were visible in the Delaware River. Faced with difficult raw water conditions and a weekend staff, the SCM was pressed into service to help achieve optimal coagulation.
The SCM can respond quickly to changing raw water and coagulant dosages to help the process control chemist decide on proper dosage changes. It can also be used to monitor the coagulation process during the 3-hour span between laboratory analysis of the plant's operating goals for pH and turbidity. With the SCM's help, Baxter's combined filter effluent remained well below Partnership goals with on-line readings ranging from 0.012 - 0.025 ntu and bench readings in the range of 0.05 - 0.06 ntu. These are normal turbidity ranges at Baxter for the on-line and bench top turbidimeters.
Conclusions
The streaming current monitor can be a useful tool in coagulant optimization. It is not a typical on-line instrument in the sense that you can't just hook it up and begin to record useful data. The SCM requires an understanding of the treatment process including the mixing dynamics and chemistry of coagulation. An individual utilities' raw water conditions and pH of coagulation will help to determine whether the SCM is going to become a useful tool for coagulant optimization or the hokus pokus machine that gives nothing but gibberish.
At the Baxter WTP the SCM was viewed as a research tool and was treated accordingly until such time that sufficient data was gathered to establish a proven track record for the instrument.
It is also important that the operators, chemists and instrumentation staff gain confidence in the unit by repeated tests to show its ability to operate properly under changing raw water conditions.
To summarize findings of the Good, The Bad and The Successful uses of the SCM:
The BAD: Sweep flocculation at high pH of coagulation.
Staff recorded either no response or very sluggish response of the SCM to this type of coagulation. At the Baxter facility the higher lime dosages and poor slaked lime mixing also caused the sensor to become dirty very quickly.
The GOOD: Coagulation at low pH below 7.0 to achieve charge neutralization.
The SCM was able to respond to minor water quality changes and small incremental coagulant dosage changes under this treatment scenario. During charge neutralization, the SCM was good at optimizing the coagulant dose, helping to determine coagulant dosages under extreme weather conditions and alerting plant personnel to chemical equipment feed failures.
The SUCCESSFUL: The Successful use of the SCM is predicated upon the following:
- Proper location of the instrument
- Routine maintenance and cleaning of sensor
- Proper sample delivery to sample cell
- Standard calibration procedure
- Protocol to establish baseline value for optimized coagulant dosage
- On-line data display to see the data trends
The ability to study the SCM trends is important for successful data interpretation. The SCM's value is in its ability to show data over a long time frame. This enables the user to develop seasonal trends and establish operating baseline values for varied raw water conditions.
About the Authors:Linda Kramer is the Water Quality Engineer and John Horger is the Plant Superintendent at the Baxter Water Treatment Plant. Kramer holds a Professional Engineer license and has received a BS and MSE from Temple University in Environmental Engineering. Questions regarding this article can be addressed to [email protected].