About the author:
Matthew Mittag is research investigator and Michael Schmidt is technical service and development for DuPont Water Solutions.
Matthew Mittag & Michael Schmidt
undefinedAs the global population continues to grow and industry develops, the sustainable supply of clean water for drinking and a wide range of other processes poses a major challenge in every corner of the world. In the face of this ever-growing demand, desalination and water reuse technologies are being deployed to ensure continued uninterrupted supply to communities and businesses with reverse osmosis (RO) offering one of the most cost-effective and efficient methods.
Unlike traditional filtration methods that use a screen or filter to extract particles, reverse osmosis (RO) is a pressure-driven process that makes use of a semipermeable membrane and the principles of crossflow filtration. Providing the finest level of filtration, the RO membrane is designed to prevent the passage of salts and organic molecules with an approximate molecular weight greater than 100. This means that an RO membrane can efficiently remove common water contaminants including bacteria, agricultural chemicals, nitrates, sugars, soluble salts and metal ions.
How Does Reverse Osmosis Work?
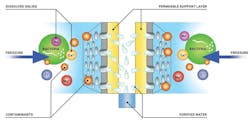
In ordinary osmosis, when a semipermeable membrane separates solutions of differing solute concentrations, the lower-concentration solution flows into the higher-concentration solution in an attempt to reach equilibrium: an equal degree of solute concentration on both sides of the membrane. As the amount of solution on the higher-concentration side increases, pressure on that water column rises until it is high enough to hinder the flow of the lower-concentration solution across the membrane. This is the action of osmotic pressure.
In RO, pressure that exceeds a system’s osmotic pressure is applied to that system. The pressure forces the higher-concentration solution back across the semipermeable membrane, leaving solutes that are blocked by the semipermeable membrane behind.
What is Crossflow Filtration?
A high-pressure pump continuously pumps feedwater into the element of an RO water treatment system. This pressure forces some water to cross the semipermeable RO membrane, resulting in a low-saline or purified product called permeate on one side, and a high-saline or concentrated brine, called concentrate or reject, on the other.
A concentrate valve controls the percentage of feedwater that goes to the concentrate stream and the permeate. In the system, the low-saline or purified permeate — the feedwater that has passed through the membrane — remains isolated from the concentrate flow. The concentrate stream removes the concentrate that cannot permeate the membrane and sweeps them out of the system.
The Anatomy of a Reverse Osmosis Membrane
A thin-film composite RO membrane is compromised of three layers, each serving a distinct purpose. The thickest layer is the support paper constructed from polyester (PET) or polypropylene (PP) which is responsible for maintaining the structural integrity of the membrane. While only approximately 100 microns thick, the support paper is able to ensure that the membrane can withstand the high pressures and temperatures that it may be subjected to during pressurisation and extended use.
The thin-film membrane layer made of polyamide (PA), which performs the separation, is the thinnest at just a quarter of a micron. By making subtle changes to the polyamide chemistry you can alter the characteristics of the solute reject.
In between these sits a support layer of polysulfone (PSf), approximately 50 microns thick. This bonds the thin-film membrane layer to the support paper while ensuring consistent, defect-free application by smoothing the coarse and irregular external surface of the support paper.
Constructing a Reverse Osmosis Element
Much like the membranes, the resulting RO element can be constructed in a variety of configurations, depending on the intended application and the desired performance profile. It is widely accepted that spiral-wound elements provide for the easiest to maintain solutions, with lower element replacement costs and a simpler plumbing system compared to tubular, plate-and-frame or hollow-fiber designs.
Elements produced using automated manufacturing processes can provide higher performance to those assembled manually due to the precise leaf set placement and uniform glue lines by maximizing the active area available to create permeate.
The precise design of an element is contingent on the intended application environment. For example, when RO elements are used for desalination, consideration needs to be given to the effective management of biofouling caused by the build-up of salt and other molecules and the resulting impact on filtration efficiency. Chemical cleaners can be used to backwash the sediment, however, these will lessen the lifespan of a membrane, so alternative solutions have been developed. You can now find RO elements with specialist anti-biofouling properties that reduce sediment build-up, increasing their overall efficiency and providing greater system uptime because of reduced need.
An Array of Applications
As noted above, RO elements can be used for desalination, providing clean water supplies for coastal communities where alternative sources of water are lacking, however, this is just one of many different applications for this water technology, which include food and beverage processing, biomedical separation and industrial process water treatment.
In addition to providing finer filtration than either nanofiltration or ultrafiltration, RO can be used as a pre-treatment process for ion exchange (IX) processes, substantially reducing the operating costs and regeneration frequency of the IX system.
There is also growing use of RO elements for wastewater treatment, facilitating greater water reuse while reducing waste discharge and allowing for the recovery of valuable by-products such as metals, salts, organic biosolids and other minerals.
While Zero Liquid Discharge (ZLD) remains the gold standard, achieving this theoretical ideal is incredibly expensive due to the thermal processes involved and can prove cost-prohibitive to many industries, especially those in developing countries. Instead, RO elements can be used in a Minimum Liquid Discharge (MLD) system that results in less than 5% of concentrated water remaining, with the remaining 95% having been transformed into purified water and salts. There is the option of subjecting the remaining concentrate to thermal pressures, effectively coupling MLD and ZLD solutions together, which can provide savings of up to 60% compared to simply using thermal ZLD.
What Does the Future Hold?
Universities and commercial R&D departments are investing resources in refining RO technology, exploring how the next generation of membranes could deliver even greater efficiencies. This includes efforts led by DuPont Water Solutions, in collaboration with Penn State, The University of Texas at Austin, Iowa State University, and Dow Chemical Company who have discovered by controlling the density of the membrane, they could improve the efficiency of an RO element by 30 to 40%, increasing water output while reducing energy consumption. Researchers mapped the path that water was taking through membranes at nanoscale, and their findings suggested that inconsistencies in density and mass distribution have a greater impact on performance than the thickness of a membrane.
While research is ongoing, it’s clear that RO elements have potential to make water purification and desalination processes more sustainable, productive and affordable, building on an already strong foundation for this water technology.