Testy History
About the author: Marianne Metzger is director of business development for National Testing Laboratories Ltd. Metzger can be reached at [email protected].
A variety of U.S. Environmental Protection Agency (EPA)-approved methods for testing total coliform and E. coli bacteria in drinking water are available. Some have been around for decades, while newer ones have been introduced to improve the process and limit false results.
There are concerns about false positives due to new, stricter regulations that could require additional testing and treatment if there are positive results, potentially leading to additional costs. Laboratories running samples for public water supplies must use an EPA-certified method. EPA allows new methods to become certified on an expedited basis, so better technology can be introduced quickly.
Century-Old Testing Methods
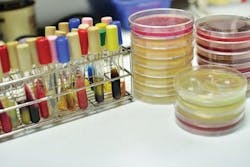
Two of the most common methods for testing coliform, fecal coliform and E. coli are most probable number (MPN) and membrane filtration. The MPN method dates back to as early as 1908 and also is known as the multiple tube or dilution tube method. As the names suggest, this method utilizes multiple tubes containing diluted samples. Each tube is given growth media, then the tubes are inverted and observed for gas formation, which indicates a positive result for coliform. The number of organisms is based on a statistical analysis of the tubes and dilutions, and the most probable number is calculated. This test is known to result in false positives due to gas production by non-coliform being interpreted as a positive for coliform.
The membrane filtration method was first used in the 1950s to compete against the MPN method. This method filters 100-mL samples through a 47-mm membrane. Any bacteria present are on the surface of the filter. The filter is put onto a petri dish with nutrients for growth and incubated for 22 to 24 hours. The dish then is examined for colonies using an epifluorescence microscope, which allows colonies to be identified and counted as coliform and E. coli.
This method provides several benefits over the MPN method, including reduced preparation time and presence or absence within 24 hours. Both test methods provide an estimated colony count. The biggest drawback to the membrane filtration method is that sometimes there could be a significant amount of non-coliform bacteria that takes over the plate, which gives an inconclusive result and invalidates the test. Membrane filtration also is known to produce a false negative result for organisms damaged by chlorine.
Enzyme Influence on Water Testing
In the 1990s, three new enzyme-based tests were evaluated and approved by EPA to meet the testing requirements of the Safe Drinking Water Act: Coliert, Coliert-18 and Colisure. They are based on a color change indicating whether coliform bacteria are present or absent.
Coliert, after 24 to 28 hours of incubation, turns yellow to indicate a positive coliform and will fluoresce when exposed to a specific wavelength of ultraviolet (UV) light. Coliert-18 only requires 18 hours of incubation for results, so testing can be completed sooner. Colisure uses a different enzyme that produces a magenta color change instead of yellow and will fluoresce when E. coli are present under UV light. The sample needs to be incubated for 24 to 48 hours. This is a good alternative method for samples that already have a yellow color due to mineral content.
Numerous other enzyme-based methods have been approved by EPA in the past 15 years. Some are similar to the three methods described above, but with improvements. Some of the methods can be used to quantify the bacteria present, yielding quantitative results. For example, the Coliert enzyme is added to the sample and poured into a tray with several small compartments and incubated for 24 to 28 hours. Much like the MPN method, the results are based on how many compartments are identified as positive and, based on a statistical analysis, an estimated colony count is given.
With some of these enzyme-based methods, false positives occur due to the presence of Aeromonas bacteria, a non-coliform. Other methods have adapted by adding antibiotics to suppress Aeromonas growth, reducing false positives.
One of the biggest drawbacks to these color change methods is that they are subjective to the analyst. Not all samples are clearly positive, and some samples may already have naturally occurring discoloration, which can interfere with the final results. The analyst may err on the side of caution, possibly identifying a negative sample as a positive result when the sample is actually negative. While false positive results can trigger additional testing and public notification, a false negative could impact public health.
New Testing Methods
In 2014, EPA approved a new method for drinking water analysis: Tecta Rapid Microbial Detection. This is an automated microbial detection system that takes most of the manual process out of traditional microbiological testing, stores the necessary paper trail electronically and emails results upon completion.
This new technology utilizes a special bottle design, which helps separate organisms into the bottom of the bottle, where the reacted organism gives off a fluorescent color based upon which organism is present after a period of incubation. Depending on the level of contamination, samples can indicate positive in as little as two to 10 hours. The unit is able to send an email when an organism is first detected, so public water supplies can react more quickly to potential outbreaks of waterborne disease. Samples are incubated for a minimum of 18 hours to get a final accurate level of detection. The unit is user-friendly and eliminates the need for a microbiologist to read sample results, thus helping reduce costs.
Microbiological testing of drinking water is essential to protecting public health. Some methods have been used for many years and are still used today as reliable methods yielding accurate results, however, advances in testing methods allow for many benefits. Method developments have resulted in lowered costs to run samples by eliminating labor-intensive preparation work and off-hour reading of plates.
Along with lower costs, newer testing methods can be run faster, yielding positive results in as little as 10 hours, with a full report available in 18 hours. That is considerably quicker than the old methods, which could take 24 to 48 hours to confirm a detection. Early notification of a positive detection can help save lives, allowing a quicker reaction when there is a potential outbreak and public health is at risk.
Finally, the latest technology introduced a system capable of providing all the required documentation relating to the running of the sample and the results without the burden of handwriting the details, which can help a laboratory when it must undergo an audit to maintain certification. Due to the electronic paper trail generated by the system, there is no way for results to be manipulated.